 National Registrar Ministry of Corporate (Govt. of India).
National Registrar Ministry of Corporate (Govt. of India). Registrar By NCT, Govt. of Delhi.
Registrar By NCT, Govt. of Delhi.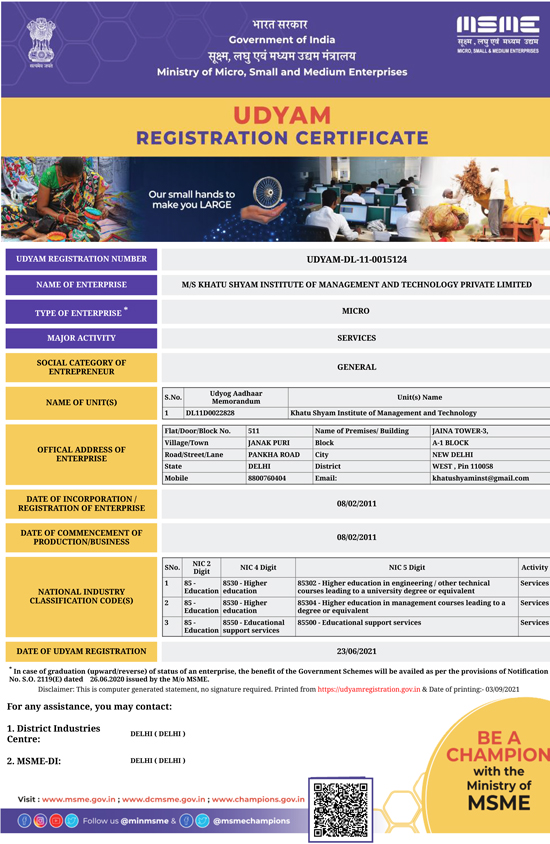 Register By Ministry of MSME, Govt. of India
Register By Ministry of MSME, Govt. of India WA 2:2007 Certified By Indraprastha SystemCert Pvt.Ltd is Accredited By Integrated Accreditation Centre For Certification (IACCA) http://www.www.iacca.in
WA 2:2007 Certified By Indraprastha SystemCert Pvt.Ltd is Accredited By Integrated Accreditation Centre For Certification (IACCA) http://www.www.iacca.in An ISO 9001-2015 Certified By Indraprastha SystemCert Pvt.Ltd is Accredited By Emirates International Accreditation Center (EIAC) https://www.eiac.gov.ae
An ISO 9001-2015 Certified By Indraprastha SystemCert Pvt.Ltd is Accredited By Emirates International Accreditation Center (EIAC) https://www.eiac.gov.ae An ISO 45001:2018 Certified By Innovative SystemCert Pvt.Ltd is Accredited By Egyptian Accreditation Council, (EGAC) http://www.egac.gov.eg
An ISO 45001:2018 Certified By Innovative SystemCert Pvt.Ltd is Accredited By Egyptian Accreditation Council, (EGAC) http://www.egac.gov.eg An ISO 14001:2015 Certified By Innovative SystemCert Pvt.Ltd is Accredited By Egyptian Accreditation Council, (EGAC) http://www.egac.gov.eg
An ISO 14001:2015 Certified By Innovative SystemCert Pvt.Ltd is Accredited By Egyptian Accreditation Council, (EGAC) http://www.egac.gov.eg
Master Program in Business Administration (Pharma and Clinical Research Management)
Lead the Healthcare Revolution with an MBA in Pharma and Clinical Research Management
The Master Program in Business Administration (Pharma and Clinical Research Management) at Khatu Shyam Institute of Management & Technology (KSIMT) is a fully theoretical, work-integrated distance learning program. Designed for pharmaceutical professionals, clinical researchers, and healthcare managers, this program focuses on pharma business strategies, clinical trial management, drug development processes, and regulatory affairs—without requiring lab work, hospital visits, or practical assignments.
Program Highlights
-
100% Distance Learning – Study flexibly from anywhere
-
Fully Theoretical Curriculum – No lab work, hospital rounds, or clinical practicals
-
Ideal for Pharma Professionals, Clinical Research Managers, and Healthcare Entrepreneurs
-
ISO Certified | Operates under Government of Delhi & Government of India Guidelines
-
Open for Students Across India and Abroad (UAE, Dubai, Qatar, Oman, Kuwait, Saudi Arabia, Bahrain)
Who Should Enroll?
This program is ideal for:
-
Pharmaceutical sales and marketing professionals
-
Clinical research coordinators and project managers
-
Regulatory affairs specialists
-
Entrepreneurs launching healthcare startups or clinical research organizations (CROs)
-
Graduates aiming for leadership in pharma and healthcare business sectors
Career Opportunities After MBA in Pharma and Clinical Research Management
Graduates from this program can pursue roles such as:
-
Pharma Business Manager
-
Clinical Research Project Manager
-
Regulatory Affairs Manager
-
Healthcare Strategy Consultant
-
Business Development Manager (Pharma/CRO)
-
Medical Affairs and Compliance Specialist
Why Choose KSIMT for Your MBA in Pharma and Clinical Research Management?
-
100% theoretical, flexible, and work-integrated learning model
-
Practical work experience considered as part of academic achievement
-
Curriculum aligned with the global pharmaceutical and clinical research industries
-
Affordable and accessible online correspondence education
-
Trusted by pharma and healthcare professionals across India and internationally
Build a Successful Career in Pharma and Clinical Research Management
Join KSIMT’s MBA in Pharma and Clinical Research Management and take the next step in leading healthcare innovations through flexible, work-integrated distance education.
Course Offered By : KSIMT under autonomous mode through distance education
Request a Callback
Admission Guidance for Working Professionals Work experience preferred (3+ years recommended)


